Molecular testing biotech Progenity NASDAQ: PROG stock was pummeled by the pandemic as its testing revenues were slashed in half. With the acceleration of COVID-19 vaccination, the maker of pregnancy and diagnostic testing kits is seeing revenues improve. The Company has repositioned itself as a biotech play betting on its Preecludia test. The test would be the first U.S. preeclampsia rule-out test. Preeclampsia affects over 700,000 women annually and is the second leading cause of maternal mortality. The Company made a strategic transformation to focus on oral delivery of biomedicals and gastrointestinal health. The Company received a U.S. patent for assessing preeclampsia using assays for free and disassociated placental growth factors. Even more promising is the granting of patents in regard to its ingestible therapeutics technology which is the Progenity Oral Biotherapeutic Delivery System. This is an ingestible capsule that can deliver large molecules to targeted areas instead of by injection. The Company severely diluted its share to raise cash and lower debt. Risk-tolerant speculators can watch for opportunistic pullbacks in shares of Progenity.
Q2 FY 2021 Business Update
On Aug. 12, 2021, Progenity provided a second-quarter 2021 business update for the period ending June 2021. The Company implemented cost-cutting that will result in nearly $97 million on annual run-rate cost savings. The Company raised $40 million in gross proceeds in June from a private placement with two healthcare investment funds. Net loss was (-$78.5 million) or (-$1.23) per share, more than double the net loss of (-$32.3 million) or (-$0.56) in the year-ago same period. Progenity CEO Harry Stylli commented, “Our GI innovation pipeline is progressing with both the Oral Biotherapeutics Delivery System and the Drug Delivery System now available as fully autonomous prototype devices that will enable key studies to be performed to advance our programs and provide potential partnership opportunities. I’m also excited by the successful outcome for the Preecludia™ PRO-104 validation study results, which we expect the independent PIs to publish soon, and we are making good progress with our single-molecule platform. I’m also pleased with the implementation and execution of our company transformation with substantial cost savings already being achieved and with more anticipated in the coming months. We are projecting multiple key catalysts in the next quarter and beyond, and we look forward to sharing those results in the near future”
Conference Call Takeaways
CEO Stylli set the tone, “As a reminder, we have effectively contracted the manufacturer of APIs, including adalimumab. Let's start with our Oral Biotherapeutics program. The goal of this program is to achieve oral delivery and to maximize systemic distribution of Biotherapeutics especially monoclonal antibodies, but also other proteins, peptides, nucleic acids, and potentially vaccines, as the GI tract is the host to the majority of the immune system. In total, we are targeting a vast $250 billion market that is primed for these oral delivery solutions. During the second quarter of 2021, we initiated preclinical studies of our lead candidates PGN-OB1 adalumimab and monoclonal which is subject to the BLA pathway and PGN-OB2, they are glutide, peptide, likely the fiber 5B2 pathway utilizing for the first time our prototype autonomous OBDS in a swine model of our own design. The goal of these studies is to demonstrate the bioavailability of our leads and utilize drug candidates within our autonomous OBDS and define its operating parameters and that of the preclinical models. Initial data with the prototype OBDS is very promising and supports the potential for the OBDS to achieve industry-leading bioavailability for proteins, peptides, and other biomolecules. Despite expected normal performance variability at this early stage of development, we recently demonstrated in a swine model, a significant drug was detected and approximately half the animals in a test group achieved an average bioavailability of approximately 15% to a maximum of 44% of IV for adalimumab following a single dose, highlighting the vast potential for this program. This is an unprecedented performance for a monoclonal antibody. We continue to make rapid progress in tweaking the prototype delivery platform, including formulations, refining our animal models, and developing our understanding of likely performance in humans. We believe that an average bioavailability of around 10% to 15% of IV with repeat dosing will prove satisfactory for a large number of biomolecules.” He concluded, “In summary, our GI innovation pipeline is progressing well both the OBDS and the DDS now available as prototype autonomous devices that are enabling key studies to be performed and advance our programs and partnership opportunities. I'm also very excited about the successful outcome from the Preecludia PRO-104 validation study and results, which we expect to be published as soon as practicable. We are also advancing our single-molecule platform and expect to have additional updates soon. In addition to the material reduction in operating expenses and tighter control of capital utilization, we anticipate multiple key catalysts this year and beyond.”
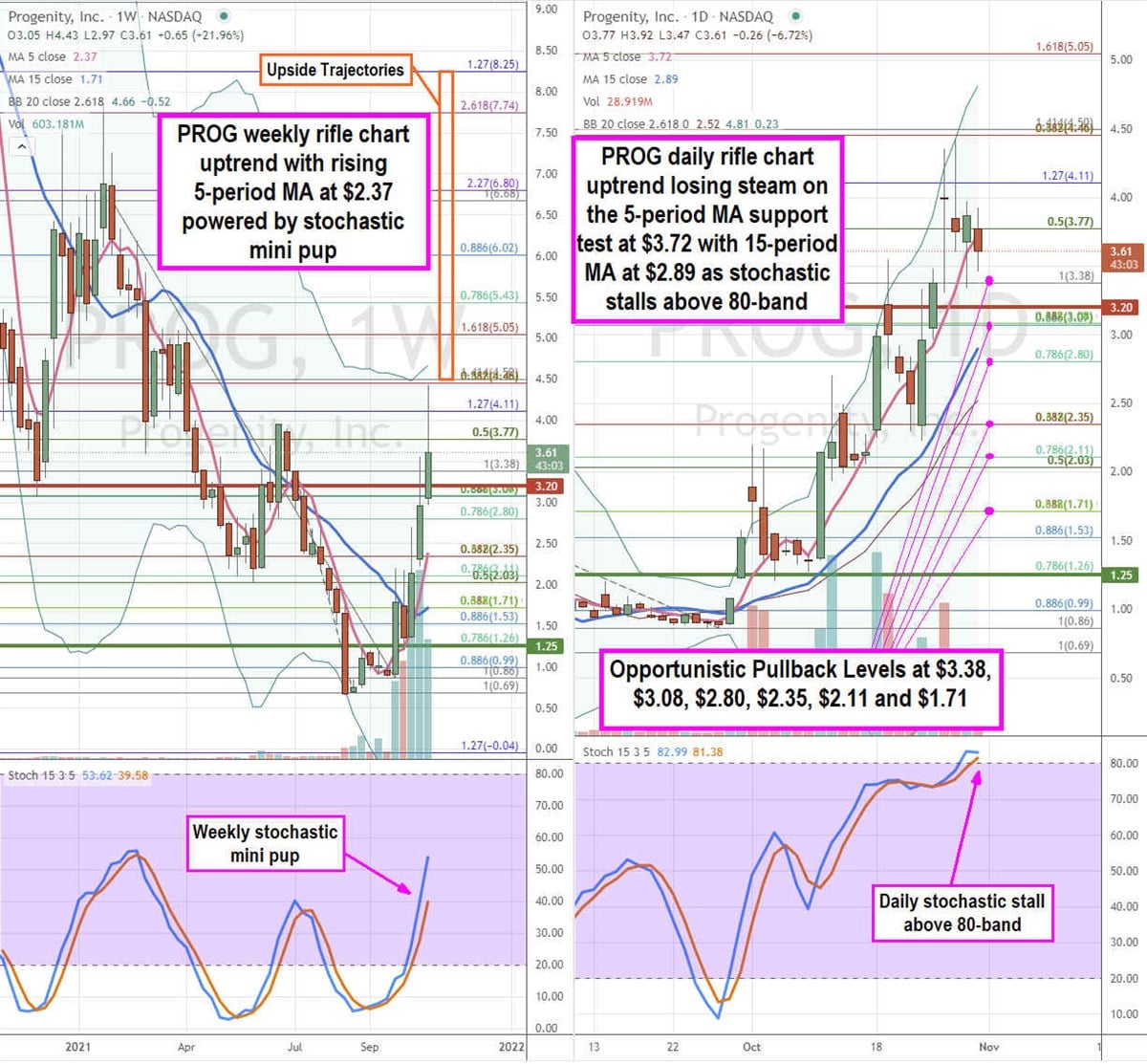
PROG Opportunistic Pullback Levels
Using the rifle charts on the weekly and daily time frames provides a precision view of the landscape for PROG stock. The weekly rifle chart peaked out near the $4.46 Fibonacci (fib) level. Shares formed a tightening reversion as the weekly 5-period simple moving average (MA) support continues to rise at $2.37 followed by the 15-period MA at $1.71. The weekly market structure low (MSL) buy triggered on a breakout through $1.25. The daily rifle chart has been in an uptrend with a rising 5-period MA at $3.72 followed by the 15-period MA at $2.89 for the channel tightening if the stochastic falls under the 80-band where its stalling flat. High-risk speculators can look for opportunistic pullback levels at the $3.38 fib, $3.08 fib, $2.80 fib, $2.11 fib, and the $1.71 fib level. Upside trajectories range from the $4.50 fib up towards the $8.25 fib.
Before you consider Biora Therapeutics, you'll want to hear this.
MarketBeat keeps track of Wall Street's top-rated and best performing research analysts and the stocks they recommend to their clients on a daily basis. MarketBeat has identified the five stocks that top analysts are quietly whispering to their clients to buy now before the broader market catches on... and Biora Therapeutics wasn't on the list.
While Biora Therapeutics currently has a Buy rating among analysts, top-rated analysts believe these five stocks are better buys.
View The Five Stocks Here
Discover the top 7 AI stocks to invest in right now. This exclusive report highlights the companies leading the AI revolution and shaping the future of technology in 2025.
Get This Free Report
Like this article? Share it with a colleague.
Link copied to clipboard.